Abstract: Introduction: intramural hematoma of the aortic wall is a component of acute aortic syndrome, and is also considered a precursor of aortic dissection. Due to peculiarities of the natural course, there are significant disagreements in choosing the optimal strategy for the treatment of intramural hematoma. Aim: was to evaluate the possibility of a differential tactical approach to the treatment of acute intramural aortic hematoma in various situations. Material and methods: two clinical cases demonstrate different approaches to the treatment of intramural aortic hematoma. Results: in given clinical examples, a conservative tactics of managing patients with intramural hematoma of the aorta "watch and wait" was applied. However, in the first case, an emergency surgical intervention was required, due to the complicated course of the disease, according to dynamic studies. The second case demonstrated the acceptability of a conservative approach with long-term monitoring of the condition of the aortic wall. Conclusions: the balance between risks of surgery and the safety of conservative therapy is the cornerstone in deciding on the optimal tactics for treating this pathology.
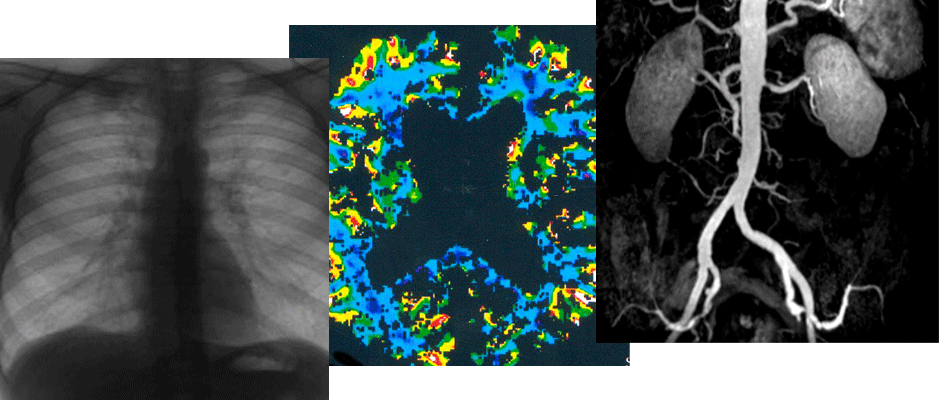
Abstract Aim: was to estimate condition of aorta branches in case of aortic dissection, using multislice computed tomography (MSCT): we estimated frequency and type of changes of main branches of the aorta involved in the dissection. Material and methods: a retrospective analysis of 104 patients with aortic dissection (AD) was performed. All patients were admitted to Scientific-Research Institute of Emergency Medicine named after N.V Sklifosovsky All studies were carried out on a multispiral (80x0.5) tomograph in early stages of the disease. Results: MSCT method allowed to obtain data of the high frequency of transition of aortic dissection to main branches (63.5%), mainly to iliac arteries (81% and 77% of aortic dissection type A and B respectively), both in isolation and in combination with other branches. However, the frequency of occurrence of hemodynamically significant stenosis, both static and dynamic, was significantly higher in groups of visceral branches and brachiocephalic arteries (82% and 71%, respectively). Conclusion: the CT method allows to evaluate in detail the lumen of the aorta and branches of aorta, and to determine type and degree of stenosis of aortic branches involved in the dissection. Revealed patterns of combining of involvement in different groups of aortic branches in the pathological process, allow to procced more optimized diagnostic search for complications of dissection, including MSCT. References 1. Hirst Ae Jr, Johns Vj Jr, Kime Sw Jr. Dissecting aneurysm of the aorta: A Review of 505 cases. Medicine (Baltimore). 1958;37(3):217-279. PMID: 13577293 https://doi.org/10.1097/00005792- 195809000-00003 2. Litmanovich D, Bankier AA, Cantin L, Raptopoulos V. Boiselle PM. CT and MRI in Diseases of the Aorta. Am J Roentgenol. 2009;193(4):928-940. PMID:19770313 https://doi.org/10.2214/ajr.08.2166 3. Wheat MW Jr. Acute dissecting aneurysms of the aorta: diagnosis and treatment-1979. Am Heart. 1980; 99(3):373-387. PMID:7355699 https://doi.org/10.1016/ 0002-8703(80)90353-1 4. Borst HG, Heinemann MK, Stone CD. Surgical treatment of aortic dissection. Churchill Livingstone International; 1996. 5. Ternovor SK, Sinitsyn VE. Spiral and electron beam angiography. Moscow: Vidar; 1998. [In Russ]. 6. Gamzaev AB ogly, Pichugin VV, Dobrotin SS. Diagnosis, surgical treatment tactics and methods for ensuring operations for aortic dissection. In: Medvedev AP, Pichugin VV. Emergency heart surgery: current and unresolved issues. Nizhny Novgorod; 2015.p.237-281. [In Russ]. 7. Belov YuV, Komarov RN, Stepanenko AB, Gens AP Savichev DD. Common sense in determining indications for surgical treatment of thoracoabdominal aortic aneurysms. Pirogov Russian Journal of Surgery. 2010;(6):16-20. [In Russ]. 8. Braverman AC. Acute Aortic Dissection. Clinician Update. Circulation. 2010; 122(2): 184-188. PMID: 20625143https://doi.org/10.1161/circulationaha.110.958975 9. Barmina TG, Zabavskaya OA, Sharifullin FA, Abakumov MM. Possibilities of spiral computed tomography in the diagnosis of damage to the thoracic aorta. Medical Visualization; 2010;(6):84-88. [In Russ]. 10. Strayer RJ, Shearer PL, Hermann LK. Evaluation, and early management of acute aortic dissection in the ED. Curr Cardiol Rev. 2012;8(2): 152-157. PMID:22708909 https://doi.org/10.2174/157340312801784970 11. Vu KN, Kaitoukov Y Morin-Roy F, Kauffmann C, Giroux MF, Therasse E, et al. Rupture signs on computed tomography, treatment, and outcome of abdominal aortic aneurysms. Insights Imaging. 2014;5(3):281-293. PM ID: 24789068 https://d0i.0rg/10.1007/s13244-014-0327-3 12. Chiu KW, Lakshminarayan R, Ettles DF. Acute aortic syndrome: CT findings. Clin Radiol.2013;68(7):741-748. PMID:23582433 https://doi.org/10.1016/j.crad.2013.03. 001 13. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Europ Heart J. 2014 35(Is 41 ):2873-2926. PMID:25173340 https://doi.org/10.1093/eurheartj/ehu281 14. Lansman SL, Saunders PC, Malekan R, Spielvogel D. Acute aortic syndrome. J Thorac Cardiovasc Surg. 2010; 140 (6Suppl): S92-97. PMID:21092805 https://doi.org/10.1016Zj.jtcvs.2010.07.062 15. Bonaca MP, O'Gara PT. Diagnosis and management of acute aortic syndromes: dissection, intramural hematoma, and penetrating aortic ulcer. Curr Cardiol Rep. 2014;16(10):536. PMID:25156302 https://doi.org/ 10.1007/s11886-014-0536-x 16. Tsai TT, Nienaber C, Eagle KA. Acute Aortic Syndromes. Circulation. 2005; 112(24): 3802-3813. PMID: 16344407 https://doi.org/10.1161/circulationaha.105.534198 17. Strayer RJ, Shearer PL, Hermann LK. Screening. evaluation, and early management of acute aortic dissection in the ED. Curr Cardiol Rev. 2012;8(2): 152-157. PMID: 22708909 https://doi.org/10.2174/ 157340312801784970 18. Husainy MA, Sayyed F, Puppala S. Acute aortic syndromepitfalls on gated and nongated CT scan. Emerg Radiol. 2016;23(4):397-403. PMID:27220654 https://doi.org/10.1007/s10140-016-1409-y 19. Olsson C, Hillebrant CG, Liska J, Lockowandt U, Eriksson P, Franco-Cereceda A. Mortality in acute type A aortic dissection: validation of the Penn classification. Ann Thorac Surg. 2011 ;92(4):1376-1382. PMID:21855849 https://doi.org/10.1016/j.athoracsur.2011.05.011 20. Kruger T, Conzelmann LO, Bonser RS, Borger MA, Czerny M, Wildhirt S, et al. Acute aortic dissection type A. Br J Surg. 2012;99( 10): 1331-1344. PMID:22961510 https://doi.org/10.1002/bjs.8840 21. Toda R, Moriyama Y Masuda H, Iguro Y Yamaoka A, Taira A. Organ malperfusion in acute aortic dissection. Jpn J Thorac Cardiovasc Surg. 2000;48(9):545-550.PMID: 11030124 https://doi.org/10.1007/bf03218198 22. Hallinan J, Anil G. Multi-detector computed tomography in the diagnosis and management of acute aortic syndromes. World J Radiol. 2014;6(6):355-365. PMID: 24976936 https://doi.org/10.4329/wjr.v6.i6.355 23. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol. 2014;72(12):1169-252. PMID:25524604 https://doi.org/10.5603/kp.2014.0225 24. Rubin GD. Helical CT angiography of the thoracic aorta. J Thorac Imaging. 1997;12(2): 128-149. PMID:9179826 https://doi.org/10.1097/00005382- 199704000-00011
Abstract: Materials and methods: 47 patients witn type В aortiс dissections underwent endovascular treatment in our departmert n 20 cases - IVUS was used for irtraoperative anatomy and morphology verification. Complications developed n 16 patients, and true lumen was reconstructed by stent-graft implantation (to cover proximal fenestration) followed by balloon-expandable stents implantation at the level of visceral arteries under IVUS control at every stage. 87,5% of patents were man, mean ago 51 8—16,2 years. References 1. Erbel R., Aboyans V., Boileau C., et al. Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35:2873-926. 2. Fattori R., Cao P., De Rango P, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013; 61: 1661-78. 3. Eggebrecht H., Nienaber C.A., Neuhauser M., et al. Endovascular stent graft placement in aortic dissection: a metaanalysis. Eur Heart J. 2006; 27: 489e98. 4. Mossop P.J., McLachlan C.S., Amukotuwa S.A., Nixon I.K. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med. 2005;2:316-21. 5. Canaud L., Faure E.M., Ozdemir B.A., Alric P., Thompson M. (2014) Systematic review of outcomes of combined proximal stent-grafting with distal bare stenting for management of aortic dissection. Ann Cardiothorac Surg. 3: 223-233. 6. Nienaber C.A., von Kodolitsch Y, Nicolas V., et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993; 328: 1-9. 7. Evangelista A., Flachskampf F.A., Erbel R., et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010; 11: 645-658. 8. Fattori R., Caldarera I., Rapezzi C., et al. Primary endoleakage in endovascular treatment of the thoracic aorta: importance of intraoperative transesophageal echocardiography. J Thorac Cardiovasc Surg. 2000; 120: 490-5. 9. Rocchi G., Lofiego C., Bigini E., et al. Transesophageal echocardiography-guided algorithm for stent-graft implantation in aortic dissection. J Vasc Surg. 2004; 40: 880-5. 10. Morton J.B., Sanders P., Sparks P.B., et al. Usefulness of phased-array intracardiac echocardiography for the assessment of left atrial mechanical “stunning” in atrial flutter and comparison with multiplane transesophageal echocardiography. Am J Cardiol. 2002; 90: 741-6. 11. Marrouche N.F., Martin D.O., Wazni O., et al. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circ 2003; 107: 2710-6. 12. Caldararu C., Balanescu S. Modern Use of Echocardiography in Transcatheter Aortic Valve Replacement: an Up-Date. M&dica. 2016; 11(4): 299-307. 13. Jongbloed MR.M., Schalij M.J., Zeppenfeld K., et al.Clinical applications of intracardiac echocardiography in interventional procedures. Heart. 2005; 91(7): 981-990. doi:10.1136/hrt.2004.050443. 14. Kang S.J., Ahn J.M., Kim W.J., et al. Intravascular ultrasound assessment of drug-eluting stent coverage of the coronary ostium and effect on outcomes. Am J Cardiol. 2013; 111: 1401-7. 15. Hitchner E., Zayed M.A., Lee G., et al. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg 2014; 59: 774-80. 16. Diethrich E.B., Irshad K., Reid D.B. Virtual histology and color intravascular ultrasound in peripheral interventions. Semin Vasc Surg. 2006; 19: 155-62. 17. Song T.K., Donayre C.E., Kopchok G.E., White R.A. Intravascular ultrasound use in the treatment of thoracoabdominal dissections, aneurysms, and transections. Semin Vasc Surg. 2006; 19: 145 9. 18. Pearce B.J., Jordan W.D. Jr. Using IVUS during EVAR and TEVAR: Improving patient outcomes. Semin Vasc Surg. 2009; 22: 172 80. 19. Lee J.T., White R.A. Basics of intravascular ultrasound: An essential tool for the endovascular surgeon. Semin Vasc Surg. 2004; 17: 110 8. 20. Gol'dina I.M., Trofimova E.Yu., Kokov L.S., Parxomenko M.V., Chernaya N.R., Sokolov V.V., Redkoborody'j A.V., Rubczov N.V. Possibilities of intravascular ultrasound examination using a phased array catheter sensor in the diagnosis and treatment of aortic dissection. Ultrazvukovaya i funktsiomalnaya diagnostika. 2016; 1: 78-89 [In Russ]. 21. Martin Z.L., Mastracci T.M. The evaluation of aortic dissections with intravascular ultrasonography. Vascular Disease Management. 2011; 03(31). Available at: http://www.vasculardiseasemanagement.com/content/ev aluation-aortic-dissections-intravascular-ultrasonography/ (accessed 10 march 2018). 22. Eggebrecht H., Nienaber C.A., Neuhauser M., et al. Endovascular stent graft placement in aortic dissection: a metaanalysis. Eur Heart J. 2006; 27: 489e98. 23. Mossop P.J., McLachlan C.S., Amukotuwa S.A., Nixon I.K. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med. 2005; 2: 316e22. 24. Nienaber C.A., Kische S., Zeller T., et al. Provisional extension to induce complete attachment after stent graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther. 2006; 13: 738e46. 25. Lombardi J.V., Cambria R.P, Nienaber C.A., et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012; 55: 629e40. 26. Hoshina K., Kato M., Miyahara T., et al. Retrospective study of intravascular ultrasound use in patients undergoing endovascular aneurysm repair: Its usefulness and a description of the procedure. Eur J Vasc Endovasc Surg. 2010; 40: 559-63. 27. Guo B-L., Shi Z-Y, Guo D-Q., et al. Effect of Intravascular Ultrasound-assisted Thoracic Endovascular Aortic Repair for «Complicated» Type B Aortic Dissection. Chinese Medical Journal. 2015; 128(17): 2322-2329.
|
authors:
|
Abstract: Aortic aneurysms and dissections are life threatening problems and pose significant management challenges. Open operative repair is associated with significant morbidity and mortality and this has prompted an increasing interest in endoluminal solutions. There are well known and potentially catastrophic complications associated with failure to achieve a seal proximally at the time of insertion and with dislocation of the prosthesis. A technique to improve fixation of the prosthesis in patients with short aortic “necks” in open and endoluminal procedures would be to staple the prosthesis to the aortic wall. A stapler would only be of value, especially for endoluminal procedures, if it could achieve transmural fixation with only endoluminal access. It became possible because of the stapler construction, containing staples made from memory-shaped metals, which can form the rings after discharge. The technology was designed by Australian company Endogene Pty. Ltd. in Russian and Australian research laboratories. The study was performed over 6 years in separate experiments on 7 adult mongrel male dogs (average weight 20 kg), 5 sheep (average weight 47 kg) and 12 pigs (average weight 68 kg). Access to abdominal aorta was obtained by central laparotomy, with the animals under general anaesthesia (sodium phentobarbital, 30 mg/kg). The deployment of the new stapler technology for graft fixation inside of animal aorta was successfully performed. The time taken for the procedure i.e., from introduction of the stapler into the aorta to removal was less than one minute. Observation of the anastomosis revealed complete staple penetration of the aortic wall and ring formation of the individual staples. There was no evidence of unexpected damage to the aortic wall and there was no bleeding at the sites of penetration of the staples through the aortic wall. In addition, there was no evidence of migration of the attached graft, or signs of thrombus formation or focal haemorrhages within the aortic wall. The Endogene Pty. Ltd. stapler technique has been successfully used in an animal model with secure graft fixation being easily obtained. Further research is required before this technology can achieve clinical application. References 1. Сутурин М.В., Григ М. Новая технология фиксации сосудистого протеза для лечения аневризмы аорты с применением внутрисосудистого степлера (экспериментальное исследование). Диагностическая и интервенционная радиология. 2008; 2 (3). 2. Slonim S.M. et al. Aortic dissection: percutaneous management of ischemic complications with endovascular stents and balloon fenestration. J. of Vasc. Surg. 1996;23: 241–253. 3. Upchurch G. et al. Endovascular Abdominal Aortic Aneurysm Repair Versus Open Repair. Why and Why Not? Pers. in Vasc. Surg. And Endovas. Ther. 2009; 21: 48–53. 4. Brewster D. et al. Long-term Outcomes After Endovascular Abdominal Aortic Aneurysm Repair. Ann. Surg. 2006; 244 (3): 426–438. 5. Leurs L. et al. Long-term Results of Endovascular Abdominal Aortic Aneurysm Treatment With the First Generation of Commercially Available Stent Grafts. Arch. Surg. 2007; 142:33–41. 6. Sun Z.J. et al. Epithelioid hemangioendothelioma of the oral cavity. Oral Dis. 2007; 13 (2):244–250.
Abstract: Authors present their first 3 cases of thoracoabdominal aneurysm hybrid repair. Endovascular procedure and open surgery were used either simultaneously, or as the steps of reconstruction. References 1. Crawford E.S., DeNatale R.W. Thoracoabdominal aortic aneurysm: Observations regarding the natural course of disease. J. Vasc. Surg. 1986; 3: 578. 2. Nienaber C.A., Eagle K.A. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. 2003; 108 (5): 628-635. 3. Kouchoukos N.T., Dougenis D. Surgery of the thoracic aorta. N. Engl. J. Med. 1997; 336: 1876-1888. 4. Meszaros I. et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117: 1271-1278. 5. Coady M.A. et al. Surgical intervention criteria for thoracic aortic aneurysms. A study of growth rates and complications. Ann. Thorac. Surg. 1999; 67: 1922. 6. Elefteriades J.A. Natural history of thoracic aortic aneurysms. Indications for surgery and surgical versus nonsurgical risks. Ann. Tho-rac.Surg. 2002; 74: 1877. 7. Lobato A.C., Puech-Leao P. Predictive factors for rupture of thoracoabdominal aortic aneurysm.J. Vasc. Surg. 1998; 27: 446. 8. Svensson L.G. et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations.J. Vasc. Surg. 1993 ;17 (2): 357-370. 9. Bavaria J. et al. Retrograde cerebral and distal aortic perfusion during ascending and thoracoabdominal aortic operations. Ann. Thorac. Surg. 1995; 60 (2): 345-353. 10. Белов Ю. В., Хамитов Ф. Ф., Генс А. П., Степаненко А. Б. Защита спинного мозга и внутренних органов в реконструктивнойхирургии аневризм нисходящего грудного и торакоабдоминального отделов аорты. Ангиология и сосудистая хирургия. 2001; 7 (4):85-95. 11. Hagan P.G. et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA. 2000; 283: 897-903. 12. FannJ.I. et al. Surgical management of aortic dissection during a 30"year period. Circulation. 1995; 92 (2): 113-121. 13. Dake M.D. et al. Endovascular stent-graft placement for the treatment of aortic dissection. New. Eng.J. Med. 1999; 340: 1546-1552. 14. Buth J. et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. Аstudy from the European сollaborators on stent-graft techniques for aortic aneurysm repair (EUROSTAR) registry. J. Vasc. Surg. 2007; 46 (6): 1103-1111. 15. Svensson L.G. et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations.J. Vasc. Surg. 1993; 17: 357-370. 16. Safi H.J. et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair. Ten years of organ protection. Ann. Vasc. Surg. 2003; 238: 372-380. 17. Chiesa R. et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J. Vasc. Surg. 2005; 42: 11-17. 18. Criado F.J., Clark N.S., Barnatan M.F. Stent graft repair in the aortic arch and descending thoracic aorta: A 4-year experience. J. Vasc. Surg. 2002; 36: 1121-1128. 19. Najibi S. et al. Endoluminal versus open treatment of descending thoracic aortic aneurysms.J. Vasc. Surg. 2002; 36: 732-737. 20. Greenberg R.K. et al. Zenith AAA endovascular graft. Intermediate-term results of the US multicenter trial. J. Vasc. Surg. 2004; 39: 1209-1218.